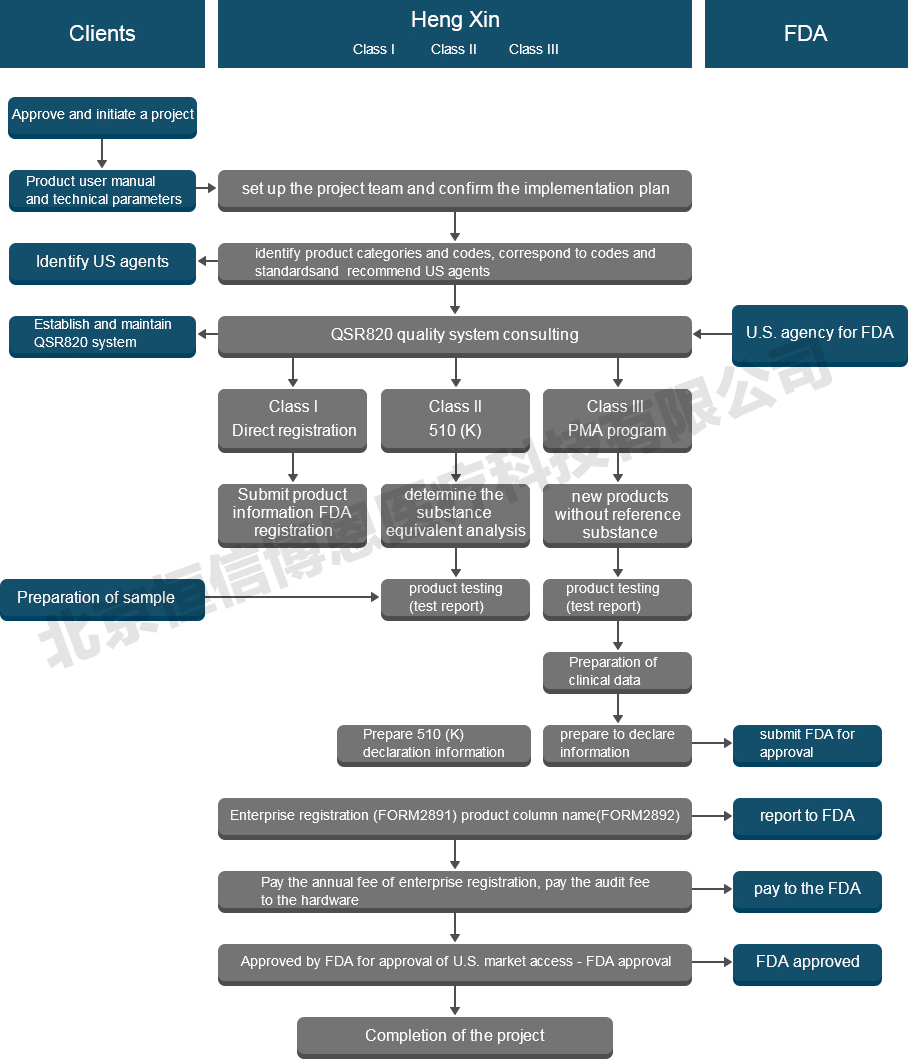
Project introduction
Most medical devices shall be submitted to the FDA pre-market notice (also known as 510 (K)), trust burns strong domestic technology ensure that in the field of human resources to China's medical enterprise to provide more professional, fast, timely and effective communication and services localization. We also provide a third-party factory simulation audit based on QSR820 requirements. Our service items are as follows:
1) Classification of medical device products according to FDA requirements
2) Provide relevant FDA regulations and guidance documents
3) Select the required listing approval procedures
4) Assist you to select an ideal domestic service agent
5) Guidance and assisstance for product testing
6) Preparation and translation of the information
7) Product registration declaration
8) Tracking of the registration process
9) Simulation audit
FDA510K