

Medical device NMPA(CFDA) Registration Process
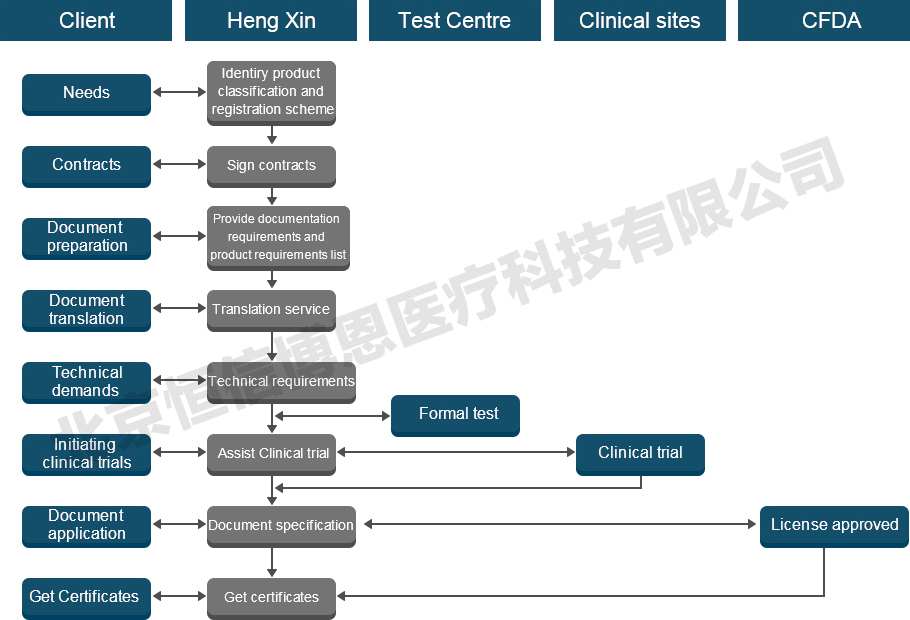
Information required
Registration information requirements and instructions for medical device
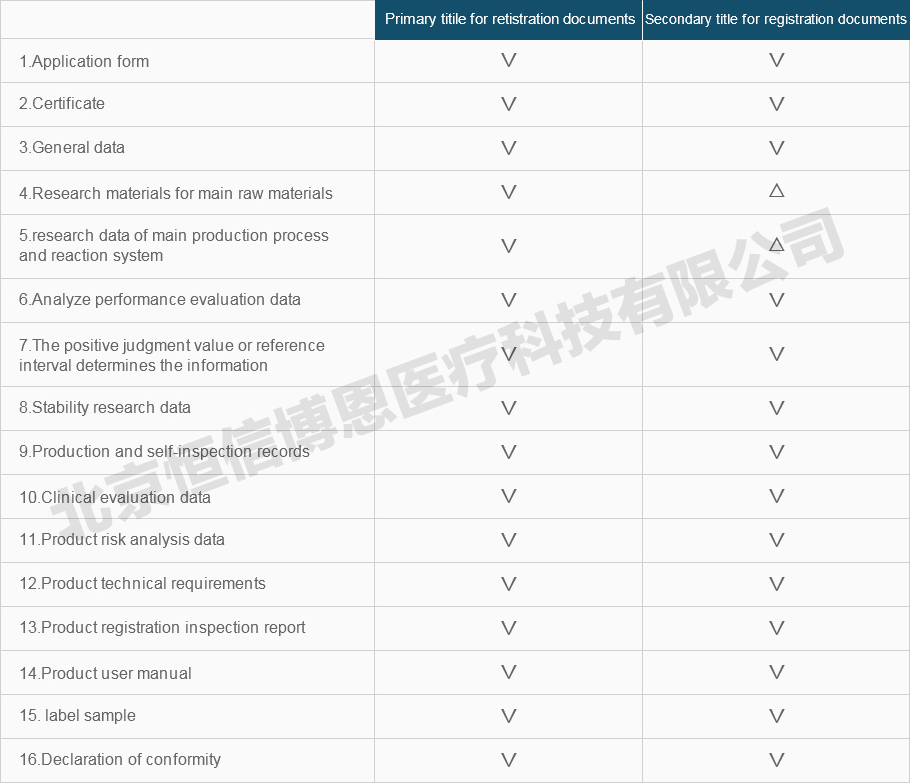
The registration information shall be submitted to the catalog, including the primary and secondary title . The documents of each secondary title should be compiled separately.