

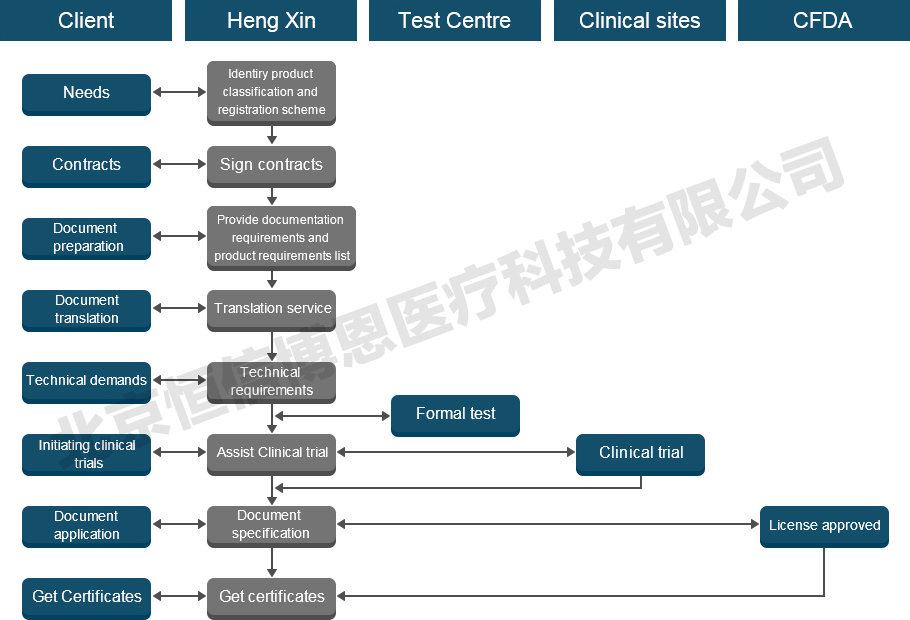
Information required
Registration information requirements and instructions for IVD products
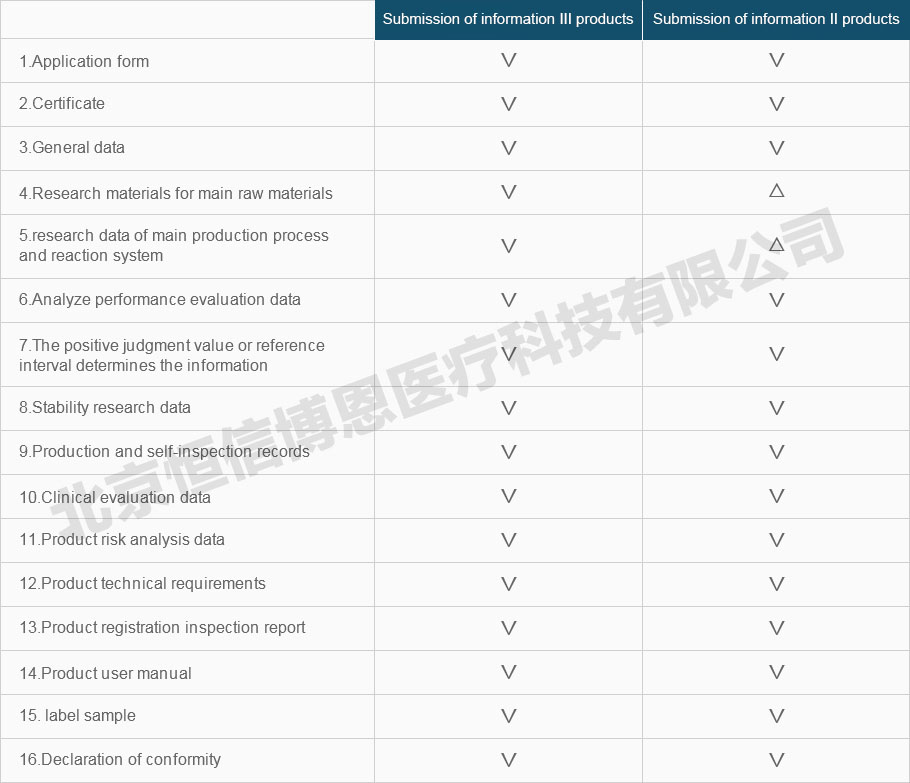
Note: the applicant should submit the declaration information according to the product category.
∨, Must provide the information.
△: No provision is required for registration application, which is maintained by the reporting unit, such as technical review.